Controllable switch for biofuel vs. bioproducts in Zymomonas mobilis
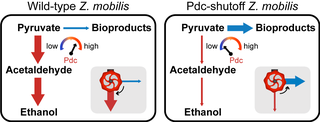
Pyruvate decarboxylase enzyme acts as a control valve for redirection of carbon along alternative metabolic pathways
The Science
The bacterium Zymomonas mobilis is an attractive target for development into a bioenergy platform organism due to its highly efficient conversion of carbon into biofuel. However, a remaining challenge is re-directing carbon away from ethanol and toward other bioproducts. To overcome this limitation, researchers at the Great Lakes Bioenergy Research Center (GLBRC) engineered strains for the ability to ramp down levels of the key ethanol-producing enzyme pyruvate decarboxylase (Pdc). This functions as a switch, allowing carbon to be funneled into other bioproducts at high efficiency.
The Impact
Production of non-native bioproducts in Z. mobilis is hampered by the fact that this organism is hard-wired to produce ethanol from pyruvate. A control switch for modulating Pdc levels will allow flexible metabolic control and future engineering of Z. mobilis to make bioproducts other than ethanol.
Summary
The pyruvate decarboxylase enzyme functions like a valve for biofuel production. When Pdc levels are high, the organism will direct more carbon toward making ethanol. When Pdc levels are low, that carbon can be shunted toward other bioproducts instead. GLBRC researchers engineered Z. mobilis pdc gene expression to respond to levels of an inducing agent. Addition of the inducing agent results in high Pdc levels and higher carbon flux toward ethanol; without the inducing agent, Pdc levels become depleted and the metabolism shifts in favor of bioproducts. Metabolomic and genetic analyses revealed that glycolytic intermediates and NADH accumulate when Pdc is depleted and that Pdc is essential for anaerobic growth of Z. mobilis. Using the Pdc control valve strategy, the researchers showed that all flux can be redirected to 2,3-butanediol under aerobic growth conditions. Anaerobically, carbon flux can be redirected to redox-balanced lactate or isobutanol pathways with ≥ 65% overall yield from glucose. The results show that, although the pdc gene is indispensable for anaerobic growth, modulating Pdc levels instills metabolic flexibility to an organism that is otherwise hard-wired to make only ethanol.
Program Manager
N. Kent Peters
Program Manager, Office of Biological and Environmental Research
kent.peters@science.doe.gov, 301-903-5549
Corresponding Author
Robert Landick
University of Wisconsin–Madison
rlandick@wisc.edu
Funding
This research was supported by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under award numbers DE-SC0018409 and DE-FC02-07ER64494.
Publication
Liu, Y., Ghosh, I.N., Martien, J., Zhang, Y., Amador-Noguez, D., & Landick, R. “Regulated redirection of central carbon flux enhances anaerobic production of bioproducts in Zymomonas mobilis.” Metabolic Engineering 61, 261-274 (2020). [DOI: 10.1016/j.ymben.2020.06.005]
Related Links
https://www.sciencedirect.com/science/article/pii/S109671762030104X