Novosphingobium aromaticivorans enzyme enables funneling of additional aromatics
Background/Objective
The plant polymer lignin is an abundant renewable source of aromatics. Chemical depolymerization yields mixtures of aromatics, including acetovanillone, a vanillin derivative with an acetyl side chain. Not all microbes that can generate chemicals from aromatics can metabolize acetovanillone, which can represent up to 10% of the aromatic monomers in deconstructed biomass.
Approach
Researchers used adaptive laboratory evolution to generate a strain of Novosphingobium aromaticivorans DSM12444 that metabolized acetovanillone and used whole-genome sequencing, in vitro aromatic phosphorylation assays, protein crystallization, computer modeling, and electron microscopy to characterize the causal mutation.
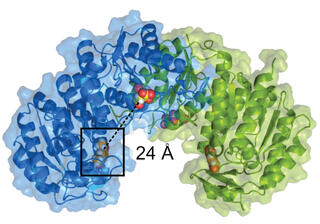
Results
This work identified a single amino acid change in a previously uncharacterized protein that is necessary and sufficient for N. aromaticivorans growth with acetovanillone as the sole organic carbon source. The variant protein, MarK E16K, phosphorylates multiple aromatic monomers, at rates 10-fold higher than known aeromatic kinases. The crystal structure predicts the E16K substitution lies in a potential ATP binding site. A search for homologs of MarK and other proteins required for acetovanillone degradation predicts this pathway for aromatic metabolism exists throughout the bacterial phylogeny.
Significance/Impact
Lignin is the most plentiful renewable source of aromatics on Earth. This work provides the first evidence of catalysis by the widespread UPF0261 protein domain, and highlights the potential of phosphorylation to increase the funneling of lignin aromatic into industrial products by wild or engineered microorganisms.
Hall, B. et al., MarK, a Novosphingobium aromaticivorans kinase required for catabolism of multiple aromatic monomers. Journal of Biological Chemistry, 110606. (2025). [DOI:10.1016/j.jbc.2025.110606]