Mimicking wood-eaters to cleave lignin for biofuels
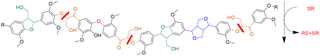
A bioinspired depolymerization strategy uses small sulfur-containing compounds to break the most common linkages in lignin
The Science
Lignin is an energy- and carbon-rich polymer found in plant cell walls. Its abundance and energy content make it a promising bio-based substitute for petroleum feedstocks for making fuels and chemicals. Breaking down the lignin into usable pieces, however, is energy intensive and costly due to the chemical recalcitrance of the linkages between its subunits. Great Lakes Bioenergy Research Center researchers have used small organic thiols (sulfur-containing compounds) to mimic enzymes from wood-digesting bacteria in an attempt to cut the bonds that hold lignin polymers together. Thiol-mediated cleavage reached up to 90% efficiency for synthetic lignin-like compounds and also resulted in a 65% molecular weight reduction for poplar lignin.
The Impact
Building on prior model-based studies, this work shows that small, diffusible thiols can penetrate complex matrices, such as biomass-derived lignin. They can access the polymer backbone and act as redox mediators to reductively cleave ether linkages, which are the most common linkages found in lignin polymers. This thiol-based strategy could be a viable way to break down lignin and enable further processing to turn it into valuable chemicals and other bioproducts.
Summary
Lignin has great potential to serve as a renewable feedstock for the production of chemicals and fuels. However, its use has been limited by the need for mild, scalable processes to depolymerize it into usable fragments. Small, organic thiols represent a bioinspired strategy to cleave the β-O-4 bond, the most common linkage in lignin. In the present study, organic thiols effectively cleaved synthetic β-O-4 linked polymers, yielding up to 90% cleaved monomer products. The thiols were also able to chemically attack lignin extracted from poplar, resulting in molecular weight reductions as high as 65% in oxidized lignin. The researchers also explored thiol-based cleavage of other types of bonds found in lignin to uncover additional potential thiol depolymerization pathways. Thiol-mediated cleavage was successful on model dimers, polymers, and biomass-derived lignin. These results illustrate the potential utility of small redox-active molecules to turn biomass-derived lignin into a variety of useful fuels and chemicals.
Program Manager
N. Kent Peters
Program Manager, Office of Biological and Environmental Research
kent.peters@science.doe.gov, 301-903-5549
Corresponding Authors
James E. Jackson
Michigan State University
jackso65@msu.edu
Eric L. Hegg
Michigan State University
erichegg@msu.edu
Funding
This material is based upon work supported by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Numbers DE-SC0018409 and DE-FC02-07ER64494.
Publications
Klinger, G.E., Zhou, Y., Foote, J.A., Wester, A.M., Cui, Y., Alherech, M., Stahl, S.S., Jackson, J.E. and Hegg, E.L., “Nucleophilic Thiols Reductively Cleave Ether Linkages in Lignin Model Polymers and Lignin,” ChemSusChem (2020). [DOI: 10.1002/cssc.202001238]
Related Links
- Link to the publication: https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.202001238
- Link to Q&A with Gracielou Klinger: https://www.glbrc.org/news/new-lignin-splitting-method-inspired-nature