Bacterium converts lignin β-5 linked aromatics into petrochemical alternatives
Background/Objective
Lignin contains aromatic subunits joined by various chemical linkages, making it challenging to produce single products from this plant polymer. Microbes can funnel lignin-derived aromatics into target chemicals, but this requires strategies to cleave major inter-unit linkages. This study showed the bacterium Novosphingobium aromaticivorans can catabolize β-5 (phenylcoumaran) linked aromatics, which account for up to 12% of interunit bonds in lignin.
Approach
Scientists with the Great Lakes Bioenergy Research Center and the Center for Bioenergy Innovation used genome-wide screens to identify candidate genes involved in catabolism of the β-5 linked aromatic dimer dehydrodiconiferyl alcohol (DC-A) followed by in vivo analysis of defined mutants and in vitro enzyme activity assays to define the pathway for its metabolism.
Results
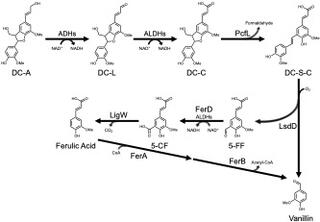
A catabolic pathway of four enzymes converts DC-A to monomers that can be funneled into products derived from the central aromatic metabolic pathway: A newly-identified γ-formaldehyde lyase, PcfL, opens the phenylcoumaran ring to form a stilbene and formaldehyde; a lignostilbene dioxygenase, LsdD, cleaves the stilbene to generate aromatic monomers vanillin and 5-formylferulate (5-FF); the aldehyde dehydorgenase FerD oxidizes 5-FF before it is decarboxylated by LigW to yield ferulic acid. Comparative genomic analysis predicts the pathway is common within the order Sphingomonadales.
Impact
Lignin is the second most abundant biopolymer on Earth and an attractive source of renewable aromatics for production of plastics, adhesives, flavorings, and other chemicals typically made from fossil fuels. This study lays the groundwork for future metabolic engineering of N. aromaticivorans and other microbes for optimized conversion of lignin into products.