The Science
Lignin, a part of plant cell walls that provides structure, contains ring-shaped molecules known as aromatics. Usually derived from crude oil, aromatics are used to make solvents, dyes, glues, medicines, and other products. Some bacteria, such as Novosphingobium aromaticivorans, can feed on aromatics from lignin and turn them into useful chemicals. But many that have been studied for this use can’t eat one common aromatic known as acetovanillone.
Using a process called adaptive laboratory evolution, scientists found that a single genetic change allows N. aromaticivorans to digest acetovanillone. DNA analysis predicts that many other bacteria use similar proteins to digest this and other related aromatic compounds.
The Impact
Lignin is the planet’s most abundant renewable source of aromatics, but more research is needed to economically turn them into useful products. This work provides the first evidence that a common but previously uncharacterized family of proteins can digest aromatics and highlights how native and engineered microbes can convert these chemicals into industrial products.
Summary
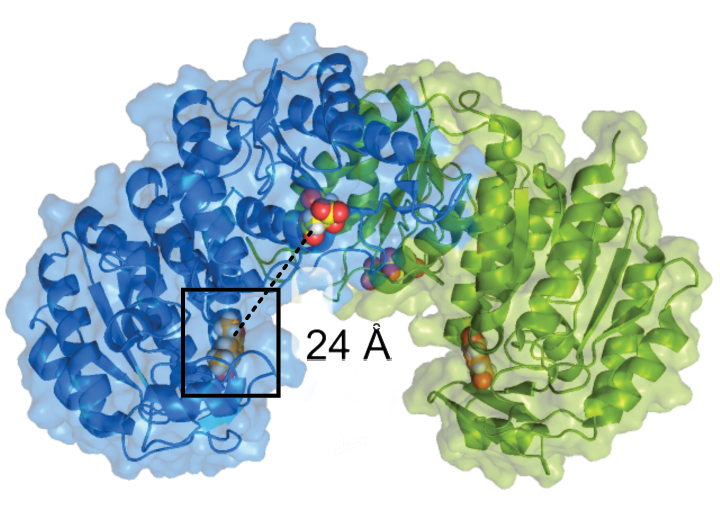
Scientists with the Great Lakes Bioenergy Research Center have developed a strain of N. aromaticivorans that converts aromatic monomers from deconstructed biomass into dicarboxylic and muconic acids and other industrial chemicals. However, the strain (DSM12444) is unable to metabolize acetovanillone, a vanillin derivative with an acetyl side chain, that can represent up to 10% of the aromatic monomers in deconstructed biomass.
Here, researchers grew cultures of DSM12444 in a mixture of acetovanillone and vanillin and gradually adjusted the ratio until acetovanillone was the sole food source. DNA analysis of the surviving microbes revealed a single mutation in the previously uncharacterized gene product. Additional experiments confirmed that the variant protein, named MarK (multiple aromatic kinase) is necessary and sufficient for N. aromaticivorans growth with acetovanillone as the sole carbon source, as well as other aromatic monomers not metabolized by wildtype cells. Structural and bioinformatic analyses predict this pathway for aromatic metabolism is widespread among bacterial species.