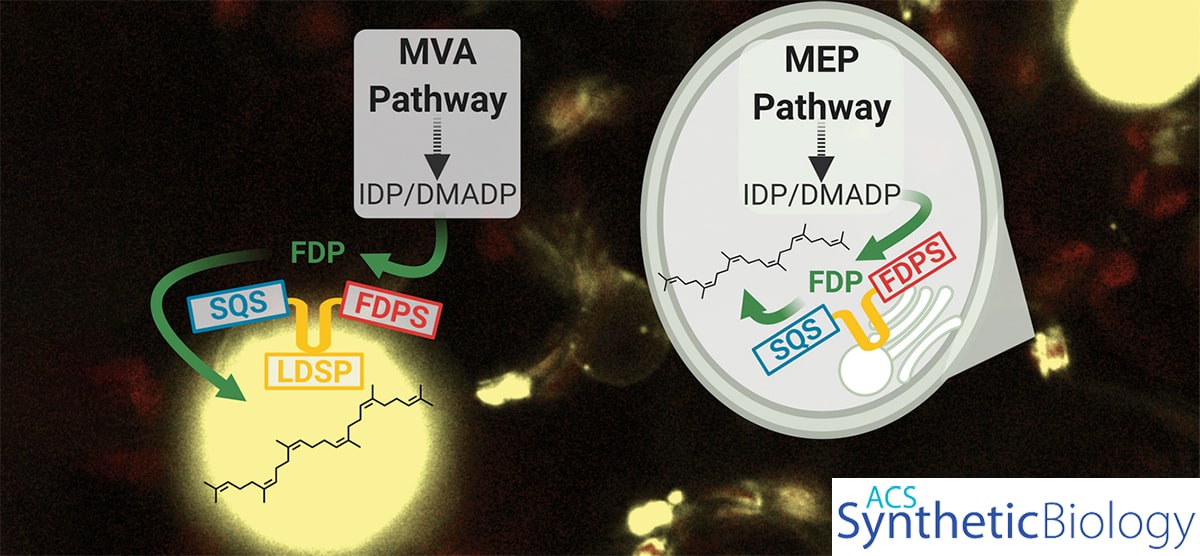
A new process developed by scientists at the Great Lakes Bioenergy Research Center enables greater production of squalene, a terpene with an array of uses in the biotechnology industry and as an energy-dense biofuel. Researchers increased plant production of the chemical by redirecting production to lipid droplets scaffolded to plastids in the cell.
The research study, “Pathway Engineering, Re-targeting, and Synthetic Scaffolding Improve the Production of Squalene in Plants,” is showcased on the cover of the most recent issue of ACS Synthetic Biology. Congratulations to the authors!
Corresponding author
Björn Hamberger | hamberge@msu.edu
Authors: Jacob D. BibikJacob D. Bibik, Sarathi M. Weraduwage, Aparajita Banerjee, Ka’shawn Robertson, Roberto Espinoza-Corral, Thomas D. Sharkey, Peter K. Lundquist, and Björn R. Hamberger
Published: June 2022, ACS Synthetic Biology
DOI: 10.1021/acssynbio.2c00051
Abstract: Plants are increasingly becoming an option for sustainable bioproduction of chemicals and complex molecules like terpenoids. The triterpene squalene has a variety of biotechnological uses and is the precursor to a diverse array of triterpenoids, but we currently lack a sustainable strategy to produce large quantities for industrial applications. Here, we further establish engineered plants as a platform for production of squalene through pathway re-targeting and membrane scaffolding. The squalene biosynthetic pathway, which natively resides in the cytosol and endoplasmic reticulum, was re-targeted to plastids, where screening of diverse variants of enzymes at key steps improved squalene yields. The highest yielding enzymes were used to create biosynthetic scaffolds on co-engineered, cytosolic lipid droplets, resulting in squalene yields up to 0.58 mg/gFW or 318% higher than a cytosolic pathway without scaffolding during transient expression. These scaffolds were also re-targeted to plastids where they associated with membranes throughout, including the formation of plastoglobules or plastidial lipid droplets. Plastid scaffolding ameliorated the negative effects of squalene biosynthesis and showed up to 345% higher rates of photosynthesis than without scaffolding. This study establishes a platform for engineering the production of squalene in plants, providing the opportunity to expand future work into production of higher-value triterpenoids.