Many people appreciate the benefits of yeast fermentation when they sip a glass of cold beer or bite into a freshly baked baguette. But for Great Lakes Bioenergy Research Center (GLBRC) researcher and University of Wisconsin-Madison assistant professor of genetics Chris Todd Hittinger, the benefits of yeast extend much farther…so far, in fact, that his yeast research could help scientists cure cancer as well as design the clean-burning “superfuels” of the future.
Hittinger’s own innovative research on yeast evolution and metabolism has earned him a string of recent successes, including a 2014 Pew Biomedical Scholar appointment and a $2 million grant from the National Science Foundation (NSF) to study yeast biodiversity. And his recent development of a novel genome-editing technique for yeast promises to accelerate the pace of his groundbreaking work.
Although Hittinger’s current research focuses on yeast evolution, it was dinosaurs that inspired his early interest in the evolutionary process. From a very young age, Hittinger was fascinated by how long ago the dinosaurs inhabited Earth and how many different types of them came and went over a period of 165 million years.
“You have this simple body plan that gets elaborated in so many different ways,” Hittinger says, “and you end up with a stunning variety of dinosaurs so exciting that almost every school child – 65 million years later! – can name them.”
It was when his middle-school biology class reached its unit on genetics that Hittinger learned there were scientific and mathematical models and theories to explain the biological diversity of dinosaurs that had always amazed him.
“I was fascinated with how quantitative aspects of biology explained genetics, how genetics could explain the way diversity is generated, and how evolution then takes place,” Hittinger remembers.
A deep interest in molecular genetics took hold, and by the time Hittinger went to college he already knew that he wanted to study the molecular basis of how biodiversity on Earth has been generated over billions of years.
Hittinger, who was born and raised in Indianapolis, Indiana, attended Southeast Missouri State University as an undergraduate, majoring in both biology and chemistry. He then entered the doctoral program at UW–Madison’s genetics department, earning his PhD in 2007, and continuing on to postdoctoral work at the Washington University School of Medicine and the University of Colorado School of Medicine. In 2011, he returned to UW–Madison as an assistant professor of genetics.
Hittinger’s early research focused on insect evolution and development, working predominantly with fruit flies.
“We were trying to understand how differences in gene sequences impact body characteristics,” Hittinger says. “For example, why do insects have only six legs when we know from the fossil record that their ancestors had many more than that?”
But the study of how evolutionary genetics works requires careful quantitative analysis of extremely small changes over time, and Hittinger and his colleagues realized that simple, single-cell fungi-like yeasts would be better model organisms for their painstakingly detailed research.
“Yeasts are single-cell organisms that have cellular organization similar to that of more complex organisms,” Hittinger explains. “Genetically, yeasts have more in common with animals than plants, which has made them extremely important to biomedical research. And because yeasts are used to convert plant sugars to ethanol through fermentation, they’re also essential to biofuels research.”
Hittinger’s team, which is composed of geneticists, microbiologists, and molecular and evolutionary biologists, is attempting to understand the genetic basis of the large variety of metabolic strategies that different species and strains of yeast adopt, as well as how these metabolic changes have evolved over time.
Hittinger’s recent $2 million grant from NSF’s Dimensions of Biodiversity Program supports research in identifying the missing links in the ancient trail of yeast evolution.
“It’s important to find missing yeast species because they help us to reconstruct evolutionary processes,” Hittinger explains. “They are the pieces of the puzzle that will help us understand not only yeast evolution but animal and human evolution as well.”
Once Hittinger has identified a new yeast species, he analyzes its metabolic strategies by looking at its growth characteristics under varying conditions (e.g., carbon food stock versus other nutrient sources) using special plate readers that can record growth for a hundred strains or conditions at once.
When a yeast of metabolic interest is identified, it undergoes genome sequencing in the lab, providing Hittinger’s team with its complete genomic make-up. Hittinger and his team then work to identify specific genes that could be useful for bioengineering synthetic yeast strains with metabolic qualities well-suited to medical, industrial, and energy purposes.
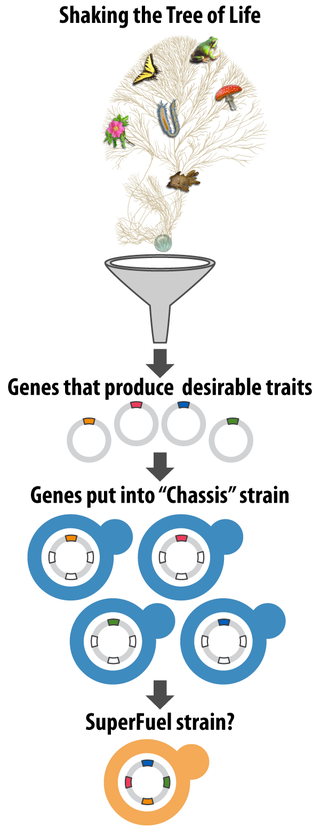
Any genes of interest are then added to, and tested in, commonly used and well-understood yeast strains (commonly called “chassis” strains).
“The idea is to shake the tree of life, get the interesting genes to fall down, and put them through a funnel into chassis strains that serve particular industrial purposes,” Hittinger says.
Because of its unique metabolic properties, Hittinger’s team is especially interested in discovering new strains of the yeast species Saccharomyces. While 90% of yeasts prefer to respire rather than ferment sugar in the presence of oxygen, somewhere along the evolutionary trail Saccharomyces acquired the preference for fermentation. Their fermentation properties, and the fact that Saccharomyces yeasts are easy to genetically manipulate, make them especially useful for Hittinger’s evolutionary research.
“Among yeasts, we see a striking array of metabolic decisions,” Hittinger says, “and we became extremely interested in this variety when we realized that cancer cells and fermenting yeasts share metabolic features.”
Hittinger’s research on the cellular resemblance between Saccharomyces yeasts and cancer cells (for which he was named a Pew Scholar), focuses on determining which steps in yeast evolution were key to making the transition from respiratory to fermentative metabolic activity, as well as the order of those evolutionary events.
“Armed with that information,” Hittinger says, “we should be able to shed some light on how cancer cells make that same transition over an individual’s lifetime.”
Hittinger’s GLBRC research focuses on promoting traits in bioengineered yeast strains that could help yeast thrive in a bio-refinery environment and more effectively convert sugars to biofuels.
“We’re looking for new biofuels that have higher energy density and can more readily drop into the existing fuel infrastructure,” Hittinger says. “Bioengineering can help us figure out how to make them.”
Hittinger’s new genome-editing technique, introduced recently in the journal Genetics, greatly enhances scientists’ ability to reach into the yeast genome and engineer precise genetic changes. The novel approach, developed with lab members William G. Alexander and Drew T. Doering, makes it possible to make 100,000 changes to a gene locus in as little as a week’s time.
“With our new strategy for genome editing,” Hittinger says, “we are able to generate and test diversity on a massive scale.”
Hittinger believes it’s the combination of these many elements of his research – finding new species, testing them, and playing with their genetic make-up – that keeps his job so interesting.
“Yeast might be a long way from dinosaurs in terms of universal appeal,” Hittinger says, “but there are many exciting evolutionary questions yet to explore. And yeast provides us with the opportunity to test our hypotheses, in precise detail, as we look for answers.”