Thermodynamics shapes the in vivo enzyme burden on glycolytic pathways
D.B. Khana et al. "Thermodynamics shapes the in vivo enzyme burden on glycolytic pathways" mBio (2025) 16:e01837-25 [DOI:10.1128/mbio.01837-25]
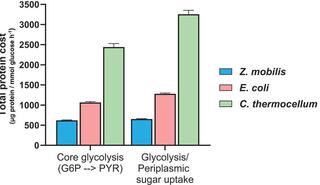
Thermodynamically constrained reactions and pathways are hypothesized to impose greater protein demands on cells, requiring higher enzyme amounts to sustain a given flux compared to those with stronger thermodynamics. To test this, we quantifiedquantifiedquantifiedthe absolute concentrations of glycolytic enzymes in three bacterial species—Zymomonas mobilis, Escherichia coli, and Clostridium thermocellum—which employ distinct glycolytic pathways with varying thermodynamic driving forces. By integrating enzyme concentration data with corresponding in vivo metabolic fluxes and ΔG measurements, we found that the highly favorable Entner-Doudoroff pathway in Z. mobilis requires only one-fourth the amount of enzymatic protein to sustain the same flux as the thermodynamically constrained pyrophosphate-dependent glycolytic pathway in C. thermocellum, with the Embden-Meyerhof-Parnas pathway in E. coli exhibiting intermediate thermodynamic favorability and enzyme demand. Across all three pathways, early reactions with stronger thermodynamic driving forces generally required lower enzyme investment than later, less favorable steps. Additionally, reflecting differences in glycolytic strategies, the highly reversible ethanol fermentation pathway in C. thermocellum requires 10-fold more protein to maintain the same flux as the irreversible, forward-driven ethanol fermentation pathway in Z. mobilis. Thus, protein investment across glycolytic pathways reflects differences in their thermodynamic favorability.
The mass spectrometry shotgun proteomics and absolute quantitation proteomics data sets for C. thermocellum, Z. mobilis, and E. coli, have been deposited to the MassIVE (Mass Spectrometry Interactive Virtual Environment) database under the accession number MSV000098743 and can be accessed at https://doi.org/10.25345/C54M91P8M.