The biosynthesis of the anti‐microbial diterpenoid leubethanol in Leucophyllum frutescens proceeds via an all‐cis prenyl intermediate
G. P. Miller et al. “The biosynthesis of the anti‐microbial diterpenoid leubethanol in Leucophyllum frutescens proceeds via an all‐cis prenyl intermediate.” The plant journal for cell and molecular biology 104 (2020) [DOI: 10.1111/tpj.14957]
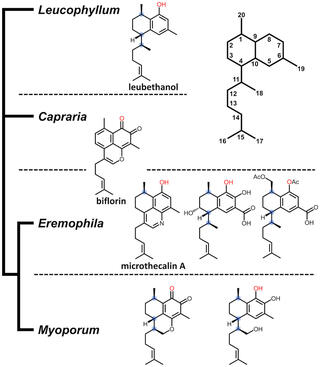
Serrulatane diterpenoids are natural products found in plants that have been characterized as having anti‐microbial properties and share a common diterpene backbone. One example, leubethanol from Texas sage (Leucophyllum frutescens) has demonstrated activity against multi‐drug‐resistant tuberculosis. Leubethanol is the only serrulatane diterpenoid identified from this genus; however, a range of such compounds have been found throughout the closely related Eremophila genus. Despite their potential therapeutic relevance, the biosynthesis of serrulatane diterpenoids has not been previously reported. Here we leverage the simple product profile and high accumulation of leubethanol in the roots of L. frutescens and compare tissue‐specific transcriptomes with existing data from Eremophila serrulata to decipher the biosynthesis of leubethanol.
Data associated with the publication is openly available on OSF. RNA‐seq data for L. frutescens have been submitted to the NCBI SRA under the accession numbers SRX8371655 (root) and SRX8371656 (flower). GenBank accession numbers for nucleotide sequences of all enzymes tested in this study are as follows: LfTPS1: MT136608; LfTPS2: MT136609; LfCPT1: MT136610; LfCPT2: MT136611; LfCPT3: MT136612; CYP706G22: MT136613; CYP76A112: MT136614; CYP736A294: MT136615; CYP736A295 : MT136616; CYP71D615: MT136617; CYP71D616: MT136618 EsTPS1: MT136619.